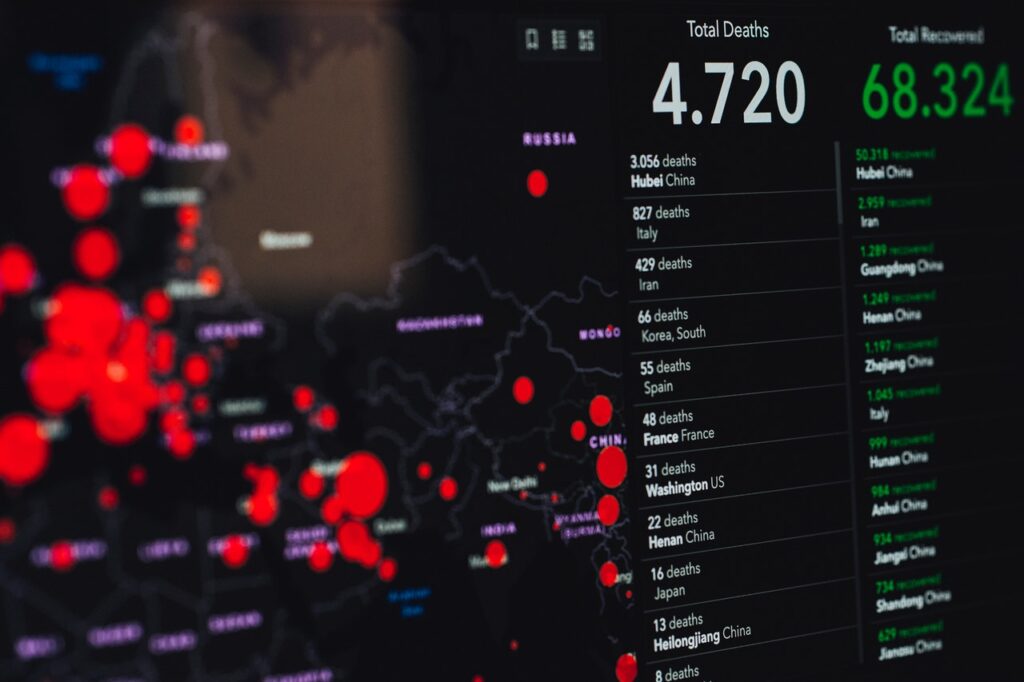
The coronavirus, or COVID 19 as it is officially known as, has greatly affect the whole of the world over the past 4 months. Now, the efforts to attempt to stop the spread of this dangerous new disease any further now lies in the hands of medical teams and researchers that are working around the clock to try and find a vaccine. There is a whole lot of urgency around the situation, so speed is of the essence to try and solve the problem. As the virus continues to spread all around the globe, research teams are going to extraordinary lengths trying to get the situation under control. We think that this is a very important topic, and we wanted to highlight some of the things that are being down to help, as well as how an ADAM Dataset system has helped astronomically in keeping things moving.

ADAM Dataset Tools Speed Up Data Entry
One of the most crucial parts of any medical testing that always takes up a lot of time and money is the data collecting stage. All of the data from each test subject and the instance of the testing must be logged and entered so that when it comes to the time to submit it to the regulators, they can determine if it is suitable or not for mass release to the public. ADAM dataset automation tools can drastically reduce the time and money that is involved in running this clinical trial process. It is much more efficient and accurate, and most importantly it is always compliant with the regulators, which means that there will be no delays in being approved.

Corona Vaccine Has Skipped Animal Testing
Whenever a medicine or vaccine is being developed and tested, it almost always goes through the animal trial stage before then moving onto human clinical trials. Although there is no direct law against skipping animal testing, it is the way it has always been done, and avoiding it completely goes against the protocol of developing a new medicine. The regulators do however state that the manufacturer must prove that it is safe before it goes into human trails, which means that it is a grey area.
Special Times Call For Special Circumstances
While it is rather unusual to skip the animal testing stage, it very much highlights the urgency that surrounds this very important issue. Industry experts are saying that if the normal route is adhered to, a coronavirus vaccine will not be seen in the market for up to 20 years. This is simply too long for the current rate of infection. A new approach was necessary in this case in order to cut down the timeline to hopefully 1 or 2 years. The arrival of the novel coronavirus was so sudden and incredibly alarming that we are having to make unprecedented changes to way we do things, whether it is in lab developing a vaccine, or our transport systems. Coronavirus will not go away anytime soon, so it essential that we act quickly.

Olivia specializes in writing helpful guides and sharing valuable information across a variety of topics.